Home
My name is Brandon Flores, and I am a student at Old Dominion University currently majoring in Exercise Science. In this website, I will be showcasing my BIOL 293 Cell Biology works completed throughout the Fall 2024 semester.
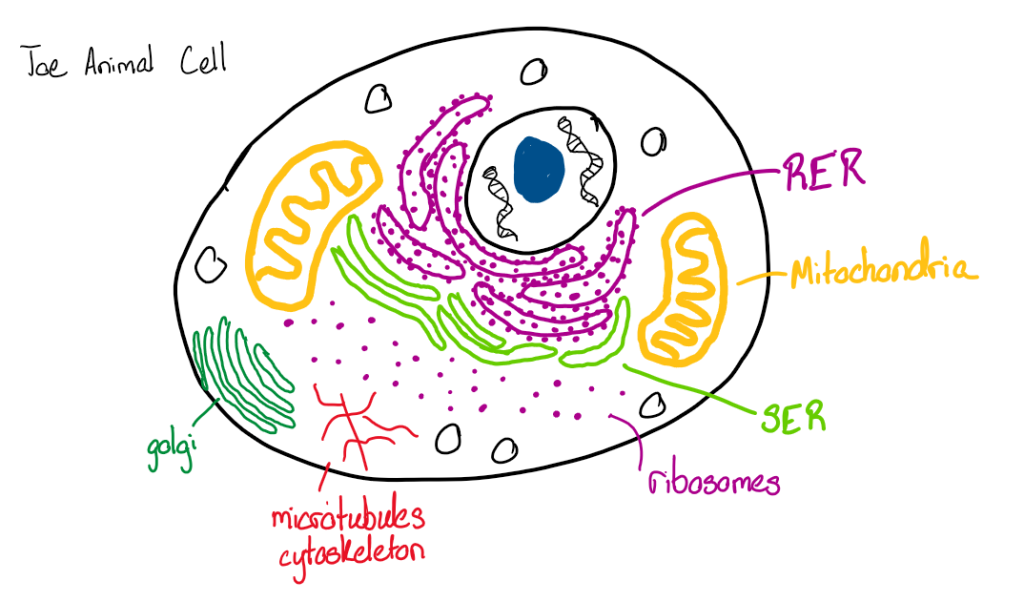
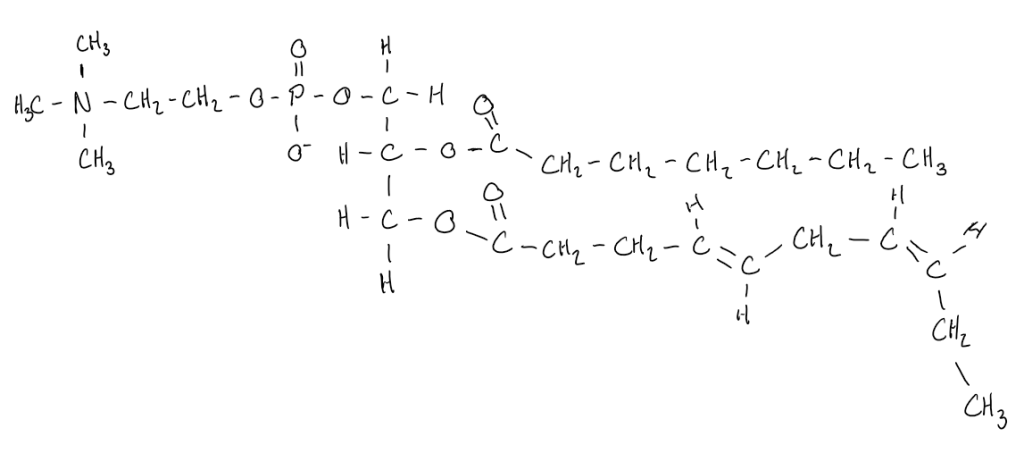

Scientific Literacy: Mitochondrial Transplant
There are over 500,000 combined cases of out-of-hospital and in-hospital cardiac arrests in the United States per year (Wikipedia). This means 0.15% of the United States population is affected by cardiac arrest every year. Of the over 350,000 annual out-of-hospital cardiac arrests, 90% of them turn out to be fatal (Latest Statistics). Some risk factors associated with cardiac arrest are age, prior incidents of cardiac arrest, and cardiovascular disease. Moreover, factors such as “cigarette smoking, high blood pressure, high cholesterol, history of arrhythmia, lack of physical exercise, obesity, diabetes, family history, cardiomyopathy, alcohol use, and possibly caffeine intake” (Wikipedia).
One of the ways cardiac injury is induced is through ischemic injury. More specifically, myocardial ischemia occurs when arterial blockages prevent arteries that supply the heart with nutrients and oxygen. Consequently, the heart’s ability to pump blood is hampered, causing abnormal heart rhythm and, if severe enough, cardiac arrest (Myocardial ischemia-Myocardial ischemia – Symptoms & causes). Reinstating blood flow to the affected tissue is mandatory for tissue survival. However, studies have shown that this can cause further injury to that tissue. This injury, ischemia-reperfusion injury, causes damage via the following mechanisms: Lack of blood flow and oxygen disrupts the electron transport chain in mitochondria and causes the cells to depend on anaerobic respiration. The relatively inefficient production of ATP and lactic acid production causes the failure of sodium-potassium pumps, failure of calcium pumps, inactivity of sodium-hydrogen exchanger pumps, and pH imbalance within the body. The failure of these pumps keeps sodium, hydrogen, and calcium ions in the cell. Consequently, water will flow into the cell to balance the high ion concentration within the cell and cause cell swelling. The high concentration of hydrogen ions also creates an acidic environment, impairing enzyme activity and causing clumping of nuclear chromatin. Post reperfusion, the influx of reactive oxygen species causes oxidative stress that damages mitochondria and cells (Wu Meng-Yu et al.). More specifically, “ROS levels will dramatically depolarize the mitochondrial membrane potential and subsequently initiate mitophagy, which is a selective autophagic process that degrades damaged mitochondria” (Liu et al.). Furthermore, tissues affected by this mitochondrial damage are more likely to be subject to energy exhaustion.
However, intercellular mitochondrial transfer research provides a promising solution to the negative effects that proceed with reperfusion. Intercellular mitochondria transport is the transfer of mitochondria from a donor cell to a recipient cell. Usually, the recipient cell is a stressed or damaged cell that exhibits reduced mitochondrial activity. According to Liu et al., J. D. McCully and his team conducted experiments involving mitochondrial transplantation to patients who suffered ischemia-reperfusion injury. The results of these experiments indicate a “reduction in the infarct size, the improvement of postinfarct cardiac function, … and improvement in their ventricular function” (Liu et al.). Given these results, mitochondrial transplantation or the promotion of intercellular mitochondria transfer from healthy donor cells provides a promising solution to ischemia-reperfusion injury.
In a study by Hayashida et al. (2023), Mitochondrial transplantation was conducted on rats and reviewed to evaluate rat survival after cardiac arrest resuscitation. The first step of this evaluation involved assessing the success of mitochondrial transplantation in an in vitro system. Neural cells with green-stained endogenous (native) mitochondria were observed after being co-cultured with red-stained exogenous (donated) mitochondria for 24 hours. The yellow staining in the merged images indicates proper integration of exogenous mitochondria into neural cells (Hayashida et al., 2023). Furthermore, this observation is consistent with exogenous mitochondria extracted from both brain and muscle tissue. The functionality of these transplanted mitochondria was assessed by measuring ATP content and mitochondria health/activity.
Figure 2A from Hayashida et al. (2023) study indicates both fresh mitochondria and frozen-thawed mitochondria are active in producing ATP after transplantation compared to the vehicle; however, fresh-mitochondria groups are approximately four times more effective than frozen-thawed mitochondria given ATP content. Figure 2B from Hayashida et al. (2023) study indicates fresh mitochondria exhibit significantly greater mitochondria membrane potential than frozen-thawed mitochondria. Because mitochondrial membrane potential is a measure of mitochondrial function/activity, it is evident fresh mitochondria are more functional and active than frozen-thawed mitochondria. Analyzing the results of major events following arrest and resuscitation in an in vivo system can provide further evaluation of the functionality of fresh mitochondria and frozen-thawed mitochondria after transplant.
Figure 3A from Hayashida et al. (2023) specifically highlights the survival rate of mitochondria-recipient rats following cardiac arrest. Both the frozen-thawed mitochondria group and vehicle group exhibited survival rates of 54.5%. On the other hand, rats that received fresh mitochondria via intravenous injection exhibited a survival rate of 90.9% (Hayashida et al., 2023). Therefore, the transplantation of fresh mitochondria nearly doubles the survival rate of rats 72 hours after cardiac arrest.
Secondly, figure 3B from Hayashida et al. (2023) indicates a relationship exists between mitochondrial transplantation in rats and neurological function following cardiac arrest. The vehicle group exhibits approximately a 60% decrease from the normal neurological assessment score of 500. Comparatively, the group of rats that received frozen-thawed mitochondria exhibited approximately a 50% decrease. Finally, the group that received fresh mitochondria only exhibited approximately a 40% decrease (Hayashida et al., 2023). Transplantation of mitochondria into animals post-cardiac arrest results in a more neurologically functional creature, with fresh mitochondria producing a greater effect than frozen-thawed mitochondria.
Figures 4A and 4B from Hayashida et al. (2023) measure blood lactose levels and lung wet: dry ratio, respectively. During cardiac arrest, the heart and lungs become dysfunctional, ceasing the delivery of oxygen to cells. As a result, mitochondria in the cells of animals must switch from aerobic respiration to anaerobic respiration. The shift to anaerobic respiration around the body will result in the significant production of lactic acid via the fermentation pathway (Steel, 2019). Analyzing Figure 4A shows that all groups returned to baseline levels of blood lactate 120 minutes after resuscitation; however, 15 minutes after resuscitation, the fresh mitochondria group exhibited approximately 2 mmol/L lower blood lactate than the frozen-thawed mitochondria group and vehicle group (Hayashida et al., 2023). The lower levels of blood lactate in the fresh mitochondria group may be indicative of tissue oxygenation or more efficient oxygen use that would delay the shift to anaerobic respiration.
Wet: dry ratio in the lungs is a way to quantitatively measure lung edema and the extent of reperfusion injury. Ischemia-reperfusion injury in the lungs causes the activation of many inflammatory pathways and, consequently, necrosis of pulmonary tissue and “significantly worsened lung function” (Ng et al., 2005). This tissue damage in the lung can cause fluid leaks in the lungs, contributing to lung edema. Analysis of Figure 4B from Hayashida et al. (2023) shows that the fresh mitochondria group had a lower value wet: dry ratio than the vehicle group and the frozen-thawed mitochondria group. Therefore, the fresh mitochondria group suffered less reperfusion injury and less pulmonary tissue damage.
After cardiac arrest, the blood glucose level in the body is generally elevated due to the release of glucose in the bloodstream – in response to stress hormones in the bloodstream – and the impaired ability of cells to uptake glucose. Figure 5C from Hayashida et al. (2023) exhibits this observation, as all groups experienced an increase in blood glucose levels 15 minutes after resuscitation. However, the blood glucose level in the fresh mitochondria group is approximately 75 mg/dL lower than the other two groups. The lower blood glucose levels of the fresh mitochondria group may be indicative of less signaling of stressor events, less deposit of stress hormones into the bloodstream, and/or less gene expression of glucose-producing (e.g., gluconeogenesis and glycogenolysis) pathway enzymes resulting from GR-cortisol-GRE binding (Steel, 2019; Kuo et al., 2015).
Lastly, Hayashida et al. (2023) Figures 7A and 7B indicate a relationship between cerebral blood flow post-cardiac arrest and transplantation of additional mitochondria. Following cardiac arrest, cerebral blood flow diminishes to approximately 80% of the baseline evident from the vehicle group. While the transplantation of frozen-thawed mitochondria shows slight recovery of cerebral blood flow after 1 hour and 2 hours, the fresh mitochondria group exhibits a much greater recovery in cerebral blood flow, seemingly even greater than baseline. Maintaining optimal cerebral blood flow is important for optimal brain function and survival.
It is also important to consider the persistence of transplanted mitochondria. In the brain, kidney, and spleen, transplanted mitochondria can be observed 1 hour and 24 hours after injection of mitochondria. However, in the heart, liver, and lungs, mitochondria were not observed after 24 hours. The absence of transplanted mitochondria may be caused by the dispersion of the mitochondria to other vital organs; however, the exact behavior is unknown as it is inconsistent across multiple studies (Hayashida et al., 2023).
Overall, fresh mitochondria should be a much greater priority over frozen mitochondria following the analyzed results. Frozen mitochondria exhibited activity and some functionality given ATP content and mitochondrial membrane potential. However, assessment of the frozen-thawed mitochondria group following cardiac arrest in an in vivo system suggests only slight differences from the vehicle group. Rat survival rate 72 hours after cardiac arrest, blood lactate levels 15 minutes after resuscitation, lung edema 72 hours after cardiac arrest, and blood glucose levels 15 minutes after resuscitation are the same or marginally different between the two groups. The only worthwhile differences between the vehicle group and the frozen-thawed mitochondria group are present in neurological function and cerebral blood flow. Finally, conclusions derived from graphs/tables throughout the paper highlight the observable benefits fresh mitochondrial transplantation has on rat survival/function post-cardiac arrest. These findings could potentially provide benefits for humans combatting cardiac arrest.
ChristinaSteelODU. (2019, Nov 9). Cytoplasmic Transport [Video]. Youtube. https://www.youtube.com/watch?v=tDuZ4emM5lo
ChristinaSteelODU. (2019, Oct 18). Glycolysis video [Video]. Youtube. https://www.youtube.com/watch?v=y-jKrDjtu-E
Hayashida K., Takegawa R., Endo Y., Yin T., Choudhary R. C., Aoki T., Nishikimi M., Murao A., Nakamura E., Shoaib M., Kuschner C., Miyara S. J., Kim J., Shinozaki K., Wang P., & Becker L. B. (2023). Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest. BMC Medicine, 21(1), 10.1186/s12916-023-02759-0
Kuo T., McQueen A., Chen T., & Wang J. (2015). Regulation of Glucose Homeostasis by Glucocorticoids. Advances in Experimental Medicine and Biology, Volume(Issue), 99-126. 10.1007/978-1-4939-2895-8_5
Latest Statistics. Sudden Cardiac Arrest Foundation, https://www.sca-aware.org/about-sudden-cardiac-arrest/latest-statistics
Liu D., Gao Y., Liu J., Huang Y., Yin J., Feng Y., Shi L., Meloni B. P., Zhang C., Zheng M., & Gao J. (2021). Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduction and Targeted Therapy, 6(1), Page. 10.1038/s41392-020-00440-z
Myocardial ischemia-Myocardial ischemia – Symptoms & causes. Mayo Clinic, https://www.mayoclinic.org/diseases-conditions/myocardial-ischemia/symptoms-causes/syc-20375417
Ng C. S. H., Wan S., & Yim A. P. C. (2005). Pulmonary ischaemia–reperfusion injury: role of apoptosis: Fig. 1—. European Respiratory Journal, 25(2), 356-363. 10.1183/09031936.05.00030304
projects C. (2002, July 5). Cardiac arrest – Wikipedia. Publication_Title, https://en.wikipedia.org/wiki/Cardiac_arrest
Wu M., Yiang G., Liao W., Tsai A. P., Cheng Y., Cheng P., Li C., & Li C. (2018). Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cellular Physiology and Biochemistry, 46(4), 1650-1667. 10.1159/000489241
Cell Biology Reflection:
Cell Biology 293 has given me the most detailed understanding of the blood glucose regulation pathways of any class. In other classes such as Anatomy and Physiology I, we touched on the pathways, but didn’t go into detail the G-Protein Coupled Receptors (GPCR) and Receptor Tyrosine Kinases (RTK) that embody them. This greater level of detail also helped me to better understand the causes and onset of Type 2 Diabetes. Now more than ever obesity, diabetes, and cardiovascular disease are prominent issues in the United States. As a future physical therapist, I can not only help myself and others through my knowledge of the body and necessary physical activity, but also through nutritional advice that specifically combats the causation of type 2 diabetes. For example, now that I know the nature of RTKs and that repeated blood glucose spikes causes eventual insulin resistance, I can make more responsible dietary decisions that don’t rise my blood glucose so intensely.
