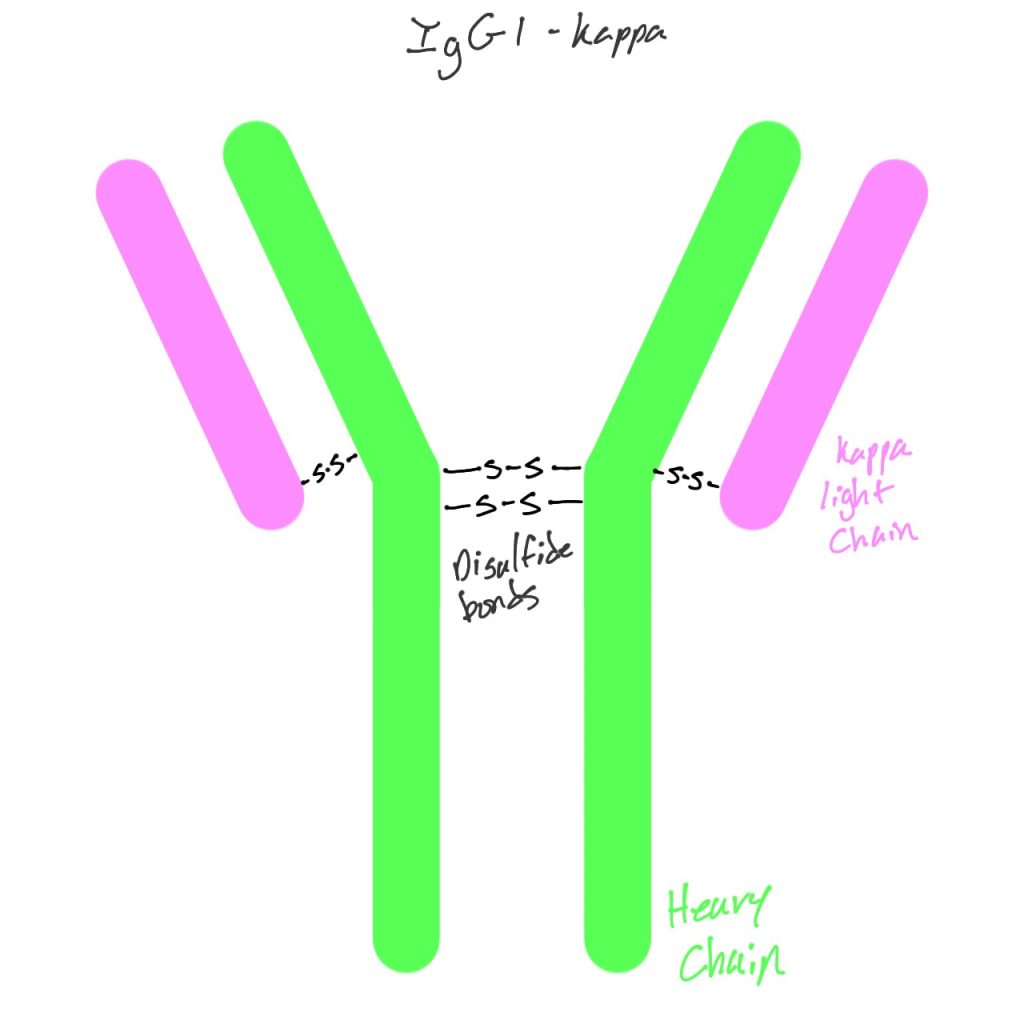
Secukinumab, also known as AIN457 and sold as Cosentyx by Novartis, is a monoclonal antibody used to treat psoriasis, psoriatic arthritis, and ankylosing spondylitis (4). It is an IgG1к antibody that binds and neutralizes IL-17A before it can interact with receptors and cause inflammation and excess growth (2).
It was discovered in 2010 (1) and approved by the FDA in 2015 (5). Secukinumab is given through either self-injections or injections by a medical provider. It is a long-term treatment with a dosage of 300mg for psoriasis and 150mg for psoriatic arthritis and ankylosing. Treatments start with injections every week for five weeks followed by an injection once a month (7). Severe side effects of the drug include an increased risk of infections, neutropenia, inflammatory bowel disease, cancer, and cardiovascular problems (3).
Psoriasis, psoriatic arthritis, and ankylosing spondylitis are caused by increased levels of interleukin 17A, a proinflammatory cytokine produced by T helper cell 17 (1). Psoriasis is an autoimmune skin disease caused by the excess creation of skin cells (6). Psoriasis can lead to psoriatic arthritis which is chronic inflammation in the joints. Ankylosing spondylitis is a condition that damages the spine by causing excess bone formation, leading to parts of the spine fusing and damage where the spine and pelvis connect (3).
- Hueber, W., Patel, D. D., Dryja, T., Wright, A. M., Koroleva, I., Bruin, G., Antoni, C., Draelos, Z., Gold, M. H., Group, P. S., Durez, P., Tak, P. P., Gomez-Reino, J. J., Study Group, R. A., Foster, C. S., Kim, R. Y., Samson, C. M., Falk, N. S., Chu, D. S., . . . Padova, F. D. (2010). Effects of AIN457, a Fully Human Antibody to Interleukin-17A, on Psoriasis, Rheumatoid Arthritis, and Uveitis. Science Translational Medicine. https://www.science.org/doi/10.1126/scitranslmed.3001107
- Patel, N. U., Vera, N. C., Shealy, E. R., Wetzel, M., & Feldman, S. R. (2017). A Review of the Use of Secukinumab for Psoriatic Arthritis. Rheumatology and Therapy, 4(2), 233-246. https://doi.org/10.1007/s40744-017-0076-0
- Eshwar, V., Kamath, A., Shastry, R., Shenoy, A. K., & Kamath, P. (2022). A Review of the Safety of Interleukin-17A Inhibitor Secukinumab. Pharmaceuticals, 15(11), 1365. https://doi.org/10.3390/ph15111365
- Secukinumab. (2024, September 2). In Wikipedia. https://en.wikipedia.org/wiki/Secukinumab
- U.S. Department of Health and Human Services (2015, February 19). Drug Approval Package. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125504Orig1s000TOC.cfm
- U.S. Department of Health and Human Services (2024, July 7). Psoriasis. National Institute of Arthritis and Musculoskeletal and Skin Diseases. https://www.niams.nih.gov/health-topics/psoriasis