Assignments from my Immunology course.
Guselkumab
Brooke Frontin
BIOL 302
10/3/2025
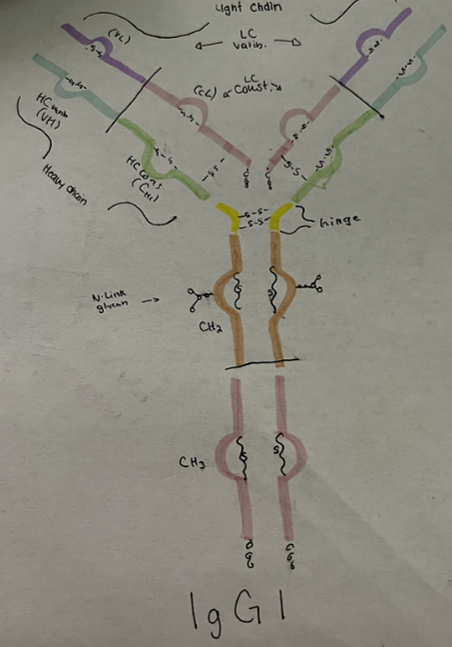
An example of a monoclonal antibody that is used in medicine today is guselkumab. Guselkumab is a drug owned and manufactured by Janssen Biotech Inc. and Johnson & Johnson. It is commercially known as Tremfya and is used to treat patients with auto immune disorders such as moderate to severe plaque psoriasis, active psoriatic arthritis, moderate to severe ulcerative colitis, and moderate to severe Crohn’s disease.The mechanism of actions in guselkumab is the human monoclonal IgG antibody. This antibody functions to create long term immunity, neutralization, and opsonization. In guselkumab, the function is to bind to the p19 subunit of interleukin 23 (IL-23) and inhibits interactions with IL-23 receptors. By inhibiting this interaction, guselkumab prevents the IL-23 receptor from producing normal proinflammatory cytokines and immune response (1).
Moderate to Severe Plaque Psoriasis
Guselkumab was originally created and approved by the FDA in November 2017 for the treatment of moderate to severe plaque psoriasis. This disease is an autoimmune disorder where the body’s immune system attacks the skin and sometimes joints. While this disease is considered hereditary, there also seems to be an interaction between genetics and environmental factors (2). Treatments categorized as biologics, like guselkumab, have appeared to be the best treatment for plaque psoriasis, but this requires long term treatment studies to be fully determined (2) The recommended dosage of guselkumab to treat severe plaque psoriasis in adults is 100mg in a subcutaneous injection. Adult patients are expected to wait 4 weeks after their first dose and then switch to every 8 weeks. Pediatric patients 6 years and older, who are also a minimum weight of 40 kg have the same documented dosage as adults. There is no limit to how long a patient can take this medication (1).
Active Psoriatic Arthritis
In July of 2020, guselkumab was approved as a treatment for active psoriatic arthritis. Active psoriatic arthritis is an autoimmune disorder where the immune system attacks the joints, and, like plaque psoriasis, the body’s immune system attacks the skin. This disease has both genetic and environmental risk factors. Genetic risk factors are genetic susceptibility in the HLA gene, and variation in the genes involved in cell signaling. Patients with psoriasis, hyperlipidemia, and who are obese, also have a much higher risk in developing psoriatic arthritis (3). The dosage of guselkumab in active psoriatic psoriasis is the same as the 100mg subcutaneous injection used by patients with plaque psoriasis. These patients also have the same injection schedule, where, after the first injection, patients wait 4 weeks, and then 8 weeks. Pediatric patients who are 6 years and older and weigh at least 40kg can take the same dosage recommended by adults. There is not a set time limit for how long these patients can take this medication (1).
Moderate to Severe Ulcerative Colitis
Guselkumab was later approved for use on moderate to severe ulcerative colitis in September of 2024. Ulcerative colitis is an autoimmune disorder characterized by the immune system attacking the colon. Risk factors associated with Ulcerative colitis are genetics, environmental factors, luminal factors, and mucosal immune dysregulation (4). Adults with moderate to severe ulcerative colitis are recommended to receive 200 mg of guselkumab via intravenous infusion for, at minimum, one hour for the first dose, then the fourth week, then every 8 weeks. If patients opt for subcutaneous injections, they will be given 400 mg of guselkumab. On week 12 and every four weeks after patients will be given 100-200mg of guselkumab. There is no limit to how long patients can take this medication (1). Moderate to Severe Crohn’s Disease As of March 2025, Guselkumab was approved for treatment on Crohn’s disease. Crohn’s disease is an autoimmune disorder where the body’s immune system attacks the gastrointestinal tract. The factors thought to contribute to this disease are the patient’s microbiome, genetics, and environmental factors. The environmental factors contributing to Crohn’s disease are often thought to be smoking, medications, and patient diet (5). Patients with Crohn’s disease have the same recommendations as patients with moderate to severe ulcerative colitis. Patients can expect to receive 200 mg of guselkumab via intravenous infusion for one hour or more for the first dose, then the fourth week, then every 8 weeks. If patients choose to use subcutaneous injections, they will be given 400 mg of guselkumab. On week 12 and every four weeks after patients will be given 100-200mg of guselkumab. There is not a time limit for how long patients can take this medication (1). Side Effects Patients who experience extreme or allergic side effects to guselkumab are recommended to immediately discontinue use if any of the following occur; dizziness, feinting, lightheadedness due to low blood pressure, swelling in the face, constricted breathing or throat tightening, tightness of the chest, itching, hives, rashes on the skin.
Works Cited:
1. Janssen Biotech, Inc. (2025, September). Tremfya (guselkumab) full prescribing information [PDF]. Retrieved October 3, 2025, from https://www.tremfya.com/?gclid=78d175fea1ad16b9e24e1d217bd3e618&gclsrc=3p.ds& utm_source=bing&utm_medium=cpc&utm_campaign=EG-DTCB-BR-NA Tremfya%20Core-JJ-Generic-Exact NA&utm_term=info%20of%20guselkumab&utm_content=Generic%20Info-TXT National-NA-1-EX 2. Sbidian, E., Chaimani, A., Garcia-Doval, I., Doney, L., Dressler, C., Hua, C., Hughes, C., Naldi, L., Afach, S., & Cleach, L. L. (2021). Systemic pharmacological treatments for chronic plaque psoriasis: A network meta‐analysis. The Cochrane Database of Systematic Reviews, 2021(4), CD011535. https://doi.org/10.1002/14651858.CD011535.pub4 3. FitzGerald, O., Alexis, O., Vinod, C., Coates, L. C., Kavanaugh, A., Tillett, W., Leung, Y. Y., Maarten, d., Scher, J. U., & Mease, P. J. (2021). Psoriatic arthritis (Primer). Nature Reviews.Disease Primers, 7(1)https://doi.org/10.1038/s41572-021-00293-y 4. Taku, K., Britta, S., Le, B. C., Wei, S. C., Ferrante, M., Shen, B., Bernstein, C. N., Silvio, D., Peyrin-Biroulet Laurent, & Toshifumi, H. (2020). Ulcerative colitis (Primer). Nature Reviews.Disease Primers, 6(1)https://doi.org/10.1038/s41572-020-0205-x 5. Cushing, K., & Higgins, P. D. R. (2021). Management of Crohn Disease: A Review. JAMA : The Journal of the American Medical Association, 325(1), 69–80. https://doi.org/10.1001/jama.2020.18936
Paper #2: T-cells and COVID Vaccine
Brooke Frontin
Old Dominion University
BIOL302
11/12/2025
Previous COVID-19 vaccination research was focused on the neutralization of antibody
responses, but currently researchers are questioning if T-cells perform a more vital role in
vaccine protection than previously realized (1). This current research is highly relevant to this
course’s content, as it elaborates on how ATP is generated in response to T-cell activation in the
SARS-Cov-2 vaccine, and how it contrasts with the previously highlighted antibody mediated
neutralization discussed in class.
According to (1), through vaccination and past infection, individuals can develop
protective immunity. Protective immunity is directed by the adaptive immune system,
maintaining both humoral immunity and cellular immunity (1). The initial response to viral
exposure is controlled by humoral immunity, which utilizes antibodies and memory B-cells that
block viral infections by binding to and neutralizing the virus before it infects host cells (1).
Many vaccines, including SARS-CoV-2, concentrate on this humoral mechanism by developing
antibodies for their respective viruses (1). It should be noted that elevated levels of antibody
concentration are required for the immune system to effectively fight viral infections (2). The
secondary response of the immune system is the cellular immune response, which is mediated by
helper CD4+ T-cells and cytotoxic CD8+ T-cells. Cellular immunity occurs if neutralization of
the virus fails and is able to infect host cells. Because of this, the response occurs rapidly to limit
the further spread of viruses in the host (1).
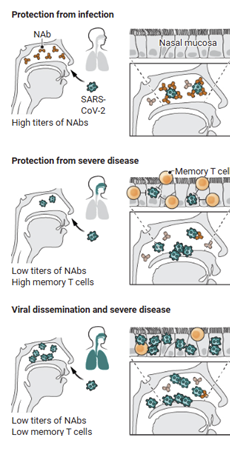
Figure 1 provides an illustration of the differences between the roles of NAbs and
memory T-cells in the SARS-CoV-2 vaccine. This Image displays how upon entrance to the
host, SARS-CoV-2 can be identified by the NAbs before infection can occur (2). It also shows
how in situations with lower numbers of NAbs, the virus can avoid recognition by NAbs. If the
virus is able to surpass neutralization, and infect host cells, they will then be thwarted by T-cell
responses. T-cell responses are facilitated by memory T-cells developed from the SARS-CoV-2
vaccine (3). If the virus is able to circumvent both immune responses due to sparse numbers or
weak responses of NAbs and T-cells, then the virus will consequently spread to the upper
respiratory tract (1).
Note. Adapted from (1)
Figure 1
Illustration of SARS-CoV-2 Immune Response Variations
There is growing interest in the data displaying the increased level of significance in the
role of T-cell responses in vaccine protection (1). The original design of vaccines was to create a
strong enough NAb response in order to entirely prevent infection and in turn suppress
transmission (1). For this to occur, vaccines need to provide high concentrations of NAbs and
effector and memory durability. Unfortunately, viral variants such as Omicron are highly
transmissible and capable of avoiding vaccine induced neutralizing antibodies (1,4,5). Because
of this, researchers have come to realize that the original goals of vaccination may not be
possible (1).
Difficulties managing Omicron continue, as it remains effective at avoiding immune
system recognition, regardless of repeated booster vaccinations. Although Omicron is largely
able to thwart the Nab immune response, it should be noted that during the Omicron surge in
South Africa, individuals with vaccines and boosters were able to maintain protection from
increasing death rates and hospitalization, regardless of the waning number of NAbs in current
vaccines (6,7). The disconnect in this data suggests that there are other mechanisms at play
allowing protection from severe variants (1).
The mechanisms used to fight viruses like Omicron have strong relevance in this course.
B-cells along with long-lived plasma cells within the germinal center undergo changes and
migrations in order to create more efficient antibodies (1). Memory B and T-cells play a vital
role in this mechanism as well, because through booster shots, individuals can increase memory
cell’s already strong durability. These cells are necessary to generate recognition in HLA
presentation scenarios because they help signal effector cells that are able to kill infected cells
directly. Although this process is typically effective, for Omicron, it is more complicated (1).
Mutations in the spike proteins of Omicron are able to inhibit antibody binding, resulting
in the incomplete neutralization of the newer viral variants (6). Although this is a major issue, the
T-cells involved in this process are capable of remaining intact and largely unimpacted by
COVID-19 variants such as Omicron (1).
Overall, the rapid development of COVID-19 vaccines and its research provided not only
a major achievement for the field of biomedical sciences, but it also provided a new perspective
on how the roles of T-cells and antibody mediated neutralization in vaccines is viewed (1). This
paper displays how ATP is generated in response to T-cell activation in vaccines or infection,
and how it is different from the antibody-mediated neutralization response.
References
- Wherry, E. J., & Barouch, D. H. (2022). T cell immunity to COVID-19 vaccines.
Science. https://doi.org/10.1126/science.add2897 - McMahan, K., Yu, J., Mercado, N. B., Loos, C., Tostanoski, L. H., Chandrashekar, A.,
Liu, J., Peter, L., Atyeo, C., Zhu, A., Bondzie, E. A., Dagotto, G., Gebre, M. S., Li, Z.,
Nampanya, F., Patel, S., Pessaint, L., Van Ry, A., Blade, K., . . . Barouch, D. H. (2021).
Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature, 590(7847),
630-634. https://doi.org/10.1038/s41586-020-03041-6 - Bange, E. M., Han, N. A., Wileyto, P., Kim, J. Y., Gouma, S., Robinson, J., Greenplate,
A. R., Hwee, M. A., Porterfield, F., Owoyemi, O., Naik, K., Zheng, C., Galantino, M.,
Weisman, A. R., Ittner, C. A., Kugler, E. M., Baxter, A. E., Oniyide, O., Agyekum, R. S.,
. . . Huang, A. C. (2021). CD8+ T cells contribute to survival in patients with COVID-19
and hematologic cancer. Nature Medicine, 27(7), 1280-1289.
https://doi.org/10.1038/s41591-021-01386-7 - Pegu, A., Schmidt, S. D., Talana, C. A., Lai, L., Albert, J., Anderson, E., Bennett, H.,
Corbett, K. S., Flach, B., Jackson, L., Leav, B., Ledgerwood, J. E., Luke, C. J.,
Makowski, M., Nason, M. C., Roberts, P. C., Roederer, M., Rebolledo, P. A., Rostad, C.
A., . . . Shi, Y. (2021). Durability of mRNA-1273 vaccine–induced antibodies against
SARS-CoV-2 variants. Science. https://doi.org/abj4176 - Collier, A. R. Y., Yu, J., McMahan, K., Liu, J., Chandrashekar, A., Maron, J. S., … &
Barouch, D. H. (2021). Differential kinetics of immune responses elicited by Covid-19
vaccines. New England Journal of Medicine, 385(21), 2010-2012. - Cele, S., Jackson, L., Khoury, D. S., Khan, K., Moyo-Gwete, T., Tegally, H., … & Sigal,
A. (2022). Omicron extensively but incompletely escapes Pfizer BNT162b2
neutralization. Nature, 602(7898), 654-656. - Gray, G., Collie, S., Goga, A., Garrett, N., Champion, J., Seocharan, I., … & Bekker, L.
G. (2022). Effectiveness of Ad26. COV2. S and BNT162b2 vaccines against Omicron
variant in South Africa. New England Journal of Medicine, 386(23), 2243-2245.
End-of-Term Reflection:
One concept I learned about in Immunology that connected to concepts from my other courses was hematopoiesis. I took Immunology concurrently with Anatomy and Physiology and Medical Terminology. In each of these courses hematopoiesis was brought up as a key part of the immunological process. Hematopoiesis is the development of new blood cells within the bone marrow. It results in the creation of red blood cells, platelets, and white blood cells that occupy most of the discussion in Immunology. Immunology was relevant to my studies in biology and my growth as a student, as it provided more depth into these topics than my other courses. In Immunology, hematopoiesis was taught as a process with distinct steps and diverging lineages, such as myeloid and lymphoid pathways.
OPTIONAL: Downloadable options for papers with images and proper formatting are shown below.