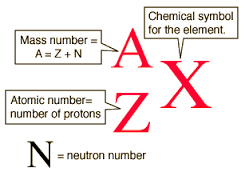Our guest blogger Rachel is back with tips from CHEM 105 Chapters 3 & 5!
 Diagnosing Your Chemistry Problems (Continued) by: Rachel Tucker
Diagnosing Your Chemistry Problems (Continued) by: Rachel Tucker
Now let’s take a look at the next set of concepts including Chapters 3 & 5 dealing with Elements and Compounds.
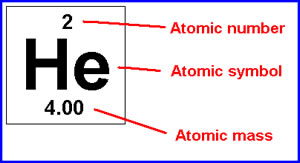
- Elements on the periodic table read with the atomic number above the symbol and the atomic mass below the symbol
- Verses single isotopes are read with the mass number (protons + neutrons) to the top left and atomic number below the symbol
- When given a periodic table, label the columns (groups) from left to right as #1-8 skipping over the transition metals. These group numbers give you all kinds of clues for finding valence electrons and typical charges.
- Group number tells you how many valence electrons the element has.
- Na = 1 valence electron
- Group numbers 1-3 have positive charges and the group # tells you what that charge is.
- Mg (group 2) = 2+ charge because when Mg bonds to other atoms, it loses 2 electrons
- Group numbers 5-7 have negative charges and 8 – the group number = the charge.
- S (group 6) = 2- charge because 8 – 6 = 2, S gains 2 electrons when it bonds to other atoms
- Elements in one group have similar bonding and chemical properties
- Group number tells you how many valence electrons the element has.
- Electron configurations
- READ THE PERIODIC TABLE LIKE A BOOK FROM LEFT TO RIGHT BEGINNING WITH HYDROGEN.
- Visualize how the periodic table is blocked out
- Groups 1-2 are the s block
- Groups 3-8 are the p block
- The transition metals are the d block
- The inner transition metals are the f block (which you do not need to worry about for this class)
- Read from left to right on each row (period), filling in the s, p, or d orbital until you have reached the end of the block
- Think of electron configurations as a blue print for building blocks – if you are given the element Cu (copper), which is element 29, every element coming before Cu (1-28) should be included in its electron configuration
- Memorize your polyatomic ions
- These interact and form compounds acting as “one unit” or “one element”
- The “ate” form of a family of polyatomic ions has more oxygen than the “ite” form
- Sulfate = SO4^-2
- Sulfite = SO3^-2
I wish you all the best in applying for your individual programs and please come by the chemistry-tutoring center in OCNPS 146 for help with any homework or study questions! We are open from 10am – 5pm M-F.