Our guest blogger Rachel is back with tips from CHEM 105 Chapter 6!
Diagnosing Your Chemistry Problems (Continued) by: Rachel Tucker
Finally, let’s explore one last section that provides a major part of the basis to mastering chemistry. This is found in Chapter 6 on Chemical Reactions.
- Balance chemical reactions by inserting coefficients. Never add or subtract from the subscripts within individual compounds when balancing.
- To find out if a redox reaction has occurred within a reaction, write out the path of each element within the reaction individually.
- Na + Cl à NaCl
- Na0 à Na1+ (sodium was oxidized, loss of an electron)
- Cl0 à Cl1- (chlorine was reduced, gain of an electron)
- HELPFUL pneumonic: OILRIG
- Oxidation is Loss (of electrons)
- Reduction is Gain (of electrons)
- Na + Cl à NaCl
- The Mole is simply a term or concept that describes quantity. This can be used interchangeably with number of molecules or number of atoms.
- When looking at an individual compound, the mole to mole relationships are found within the subscripts
- C6H9O2 – for every 1 mole of this compound, there are 6 moles of C, 9 moles of H, and 2 moles of O
- When looking at a chemical reaction, the mole to mole relationships are found within the coefficients
- Zn + 2 HCl à ZnCl2 + H2 – 1 mole of Zn will react with 2 moles HCl to produce 1 mole of ZnCl2 and 1 mole H2
- When looking at an individual compound, the mole to mole relationships are found within the subscripts
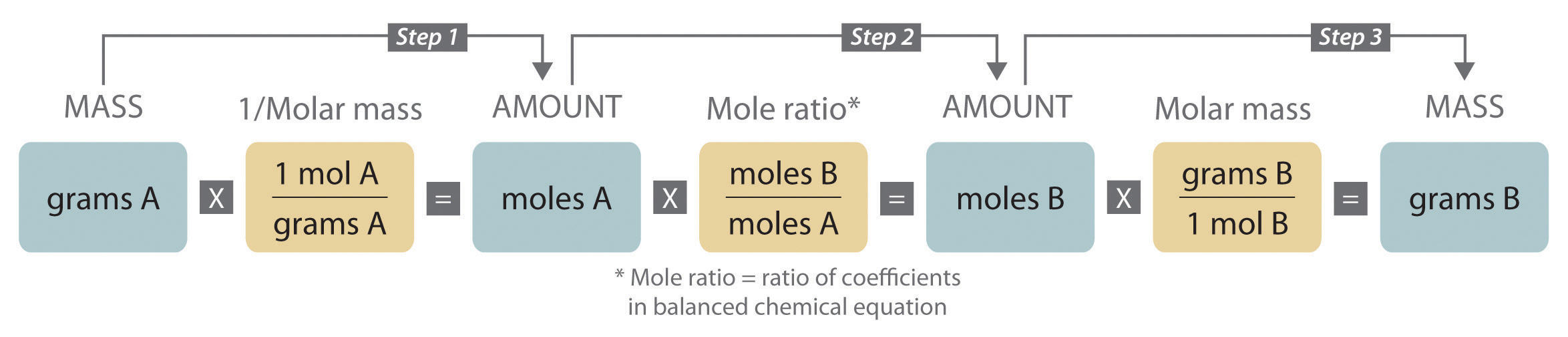
- Remember, when going from grams to grams, you MUST use your mole-to-mole factor derived from your chemical equation. Here’s a helpful roadmap:
**For a helpful resource in finding concepts and terms described or defined in a different way, a list of different conversion factors, and a very easy to use periodic table consult the following website as a source: http://chemwiki.ucdavis.edu
Well I know that was a lot of information but all very key concepts to understanding CHEM 105. Practice is key, but practicing the right way is even more important. Again, these are just some highlights and building blocks from the major chapters in introductory chemistry.
I wish you all the best in applying for your individual programs and please come by the chemistry-tutoring center in OCNPS 146 for help with any homework or study questions! We are open from 10am – 5pm M-F.