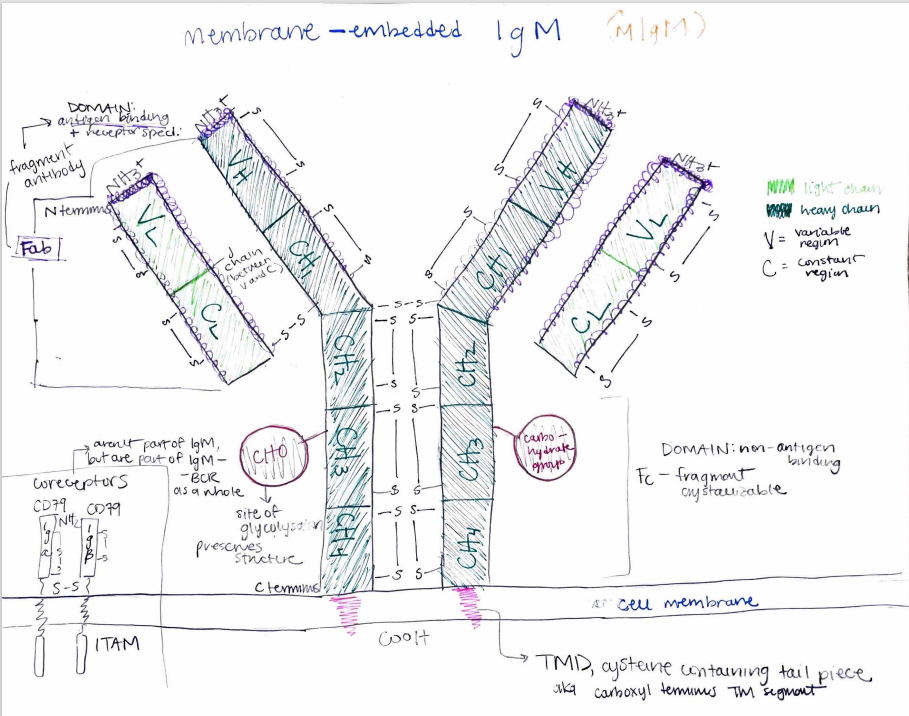
Human immunoglobulin M (IgM) is an isotype of immunoglobulin, or antibody found in humans. The domains of immunoglobulins serve as immune receptors, and as such, membrane-embedded IgM (mIgM) serves as a B-cell receptor (BCR). Expressed by mature, naive B cells, mIgM purports to activate B-cell effectors through its activation via contact with antigens, and thus must be structured both constantly and variably to function both as a consistent and antigen-specific BCR. This particular sketch of an mIgM illustrates its quaternary structure, with two identical light chains (light green) and two identical heavy chains. These chains, surrounded by disulfide bonds, consist of variable (VH, VL) and constant (CH1-4, CL) regions that denote function; while constant regions are constant throughout all IgMs, the variable loops near the outermost N-terminal are receptor specific for antigen-binding. The “top” branches of all 4 chains (VL, CL, VH, CH1) form the fragment antibody, or Fab domain, form such specificity, and as they provide location for antigen binding. The remaining constant regions form the fragment crystallizable, or Fc, which forms the non-antigen binding domain. Moving down the IgM are the carbohydrate groups (CHO), which are sites of glycosylation and provisions of structure. Following through the cell membrane, to which the IgM is embedded, is the carboxyl terminal transmembrane segment (TMD), or a cysteine-containing tail piece that connects the IgM to intracellular effector proteins. In the bottom left corner of the drawing are the Ig𝛂 and Ig𝛽 coreceptors; although they are not directly part of the immunoglobulin, they are part of the IgM-BCR as a whole.