Find me an -mAb: Lecanemab
Lecanemab, now sold under the brand name Leqembi, is a novel monoclonal antibody recently approved in the US for the treatment of Alzheimer’s disease. Following a Phase III clinical trial published in the January 5th, 2023 The New England Journal of Medicine, Biogen Inc.’s new mAb was approved via the FDA’s accelerated pathway for approval of diseases with unmet medical needs (Macmillan, 2023). The targeted disease, Alzheimer’s (AD), is a progressive neurodegenerative disease associated with cognitive decline and memory deficiency (Deture & Dickson, 2019). Though the specific causes of Alzheimer’s are not completely understood, it is known that characteristic changes in the brain result in the loss of neurons and neural connection. According to the FDA (2023), amyloid-beta plaques and neurofibrillary tangles, specifically, result in the loss of neurons and thus the diminished functions of memory and cognition. AD is usually denoted as a sporadic condition, but researchers have identified genetic risk factors associated with onset. According to Deture and Dickson (2019), the disease is thought to be caused by mutations in the presenilin 1 or 2 (PSEN1, PSEN2) genes located on chromosome 21, which are responsible for the production of amyloid precursor protein (APP). As upheld by the National Institute on Aging (2022), such mutation results in the accumulation of amyloid-beta (Aβ) plaques, and it is believed that abnormal levels of Aβ clump to form plaque buildup between neurons, resulting in the disruption of cell function. Upon clinical testing, Lecanemab was found to slow clinical decline by 27% after 18 months of treatment through the reduction of Aβ plaque (Macmillan, 2023). The novel drug is an immunoglobulin G, specifically IgG2a.
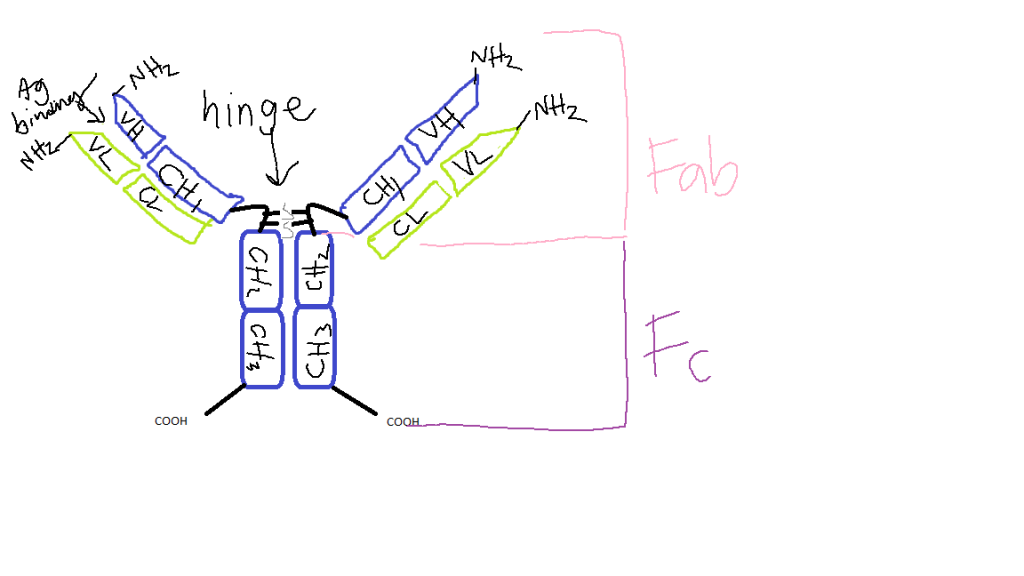
Though Aβ can have positive effects in neural tissue, including the prevention of cytotoxic damage and enhancement of plasticity, it can also be toxic, such as in the case of toxic buildup found in Alzheimer’s (Carrillo-Mora et al, 2014). Aβ can disrupt cell signaling in a multitude of ways, including disruption of insulin receptors, MAP kinases, and TLRs, which can lead to lipid and protein peroxidation and mitochondrial dysfunction via the extraction of protons from nearby lipids or proteins. According to Biogen in 2023, Lecanemab, however, “selectively binds to neutralize and eliminate soluble, toxic amyloid-beta (Aβ) aggregates that are thought to contribute to the neurodegenerative process in AD”. Formally known as mAb158, Lecanemab works to degrade Aβ protofibrils. The mAb itself is produced by first cleaving IgG at a site below the hinge region, followed by the removal of Fc fragments and non-cleaved antibody from Fab2 fragments, which results in IgG2a or RmAb158 (Solivander et al, 2018). Lecanemab then is able to recognize epitopes 1-16 of Aβ, as well as 21-29. It is on epitope 22 that the mutation of APP occurs, which results in formation of protofibrils on Aβ, and is thought to result in the aggregation of Aβ plaque in cases of Alzheimer’s. mAb158 degrades these protofibrils, which eliminates some of the Aβ plaque via reduction of accumulation, thereby restoring some of the neuronal tissue function and halting some neurodegeneration.
References
Carrillo-Mora, P., Luna, R., & Colín-Barenque, L. (2014). Amyloid beta: Multiple mechanisms of toxicity and only some protective effects? Oxidative Medicine and Cellular Longevity, 2014, 1–15. https://doi.org/10.1155/2014/795375
Commissioner, O. of the. (2023, January 6). FDA grants accelerated approval for alzheimer’s disease treatment. U.S. Food and Drug Administration. Retrieved February 5, 2023, from https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment
DeTure, M. A., & Dickson, D. W. (2019). The neuropathological diagnosis of alzheimer’s disease. Molecular Neurodegeneration, 14(1). https://doi.org/10.1186/s13024-019-0333-5
LECANEMAB confirmatory phase 3 clarity ad study met primary endpoint, showing highly statistically significant reduction of clinical decline in large global clinical study of 1,795 participants with early alzheimer’s disease. Biogen. (2023, January). Retrieved February 5, 2023, from https://investors.biogen.com/news-releases/news-release-details/lecanemab-confirmatory-phase-3-clarity-ad-study-met-primary
MacMillan, C. (2023, January 19). Lecanemab, the new alzheimer’s treatment: 3 things to know. Yale Medicine. Retrieved February 5, 2023, from https://www.yalemedicine.org/news/lecanemab-leqembi-new-alzheimers-drug
Söllvander, S., Nikitidou, E., Gallasch, L., Zyśk, M., Söderberg, L., Sehlin, D., Lannfelt, L., & Erlandsson, A. (2018). The AΒ protofibril selective antibody MAB158 prevents accumulation of AΒ in astrocytes and rescues neurons from AΒ-induced cell death. Journal of Neuroinflammation, 15(1). https://doi.org/10.1186/s12974-018-1134-4
U.S. Department of Health and Human Services. (n.d.). What happens to the brain in alzheimer’s disease? National Institute on Aging. Retrieved February 5, 2023, from https://www.nia.nih.gov/health/what-happens-brain-alzheimers-disease