ePortfolio # 1: Find me a -mAb
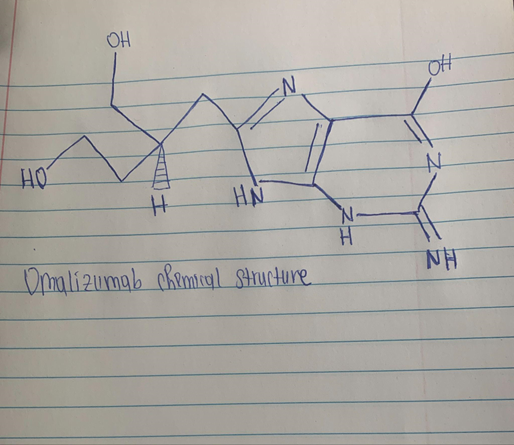
Omalizumab is a recombinant humanized immunoglobulin (IgG1) that binds IgE with great affinity created to treat allergic conditions (1). One of the conditions this drug treats is asthma, which is a chronic condition that affects the airways in the lungs (2). Omalizumab also known as Xolair is manufactured by Genentech, a member of the Roche Group (3). Omalizumab was approved by the U.S. Food and Drug Administration (FDA) to treat tolerable to acute asthma. The suggested dose of omalizumab for treating asthma is 75 to 375 mg every two or four weeks via injection under the skin. The patient’s weight and total IgE serum levels are considered while determining the right omalizumab dosage and frequency. Omalizumab works directly by attaching especially to the C-ɛ-3 locus, which is the site where IgE attaches to FCɛRI. By blocking the antibody from binding with its high-affinity receptor, which is mostly found on eosinophils, basophils, and mast cells, this action efficiently reduces immunoglobulin levels. The allergic reaction is interrupted by this. Additionally, the overall amount of free IgE is reduced, which reduces the production of the IgE high-affinity receptor on cells that cause inflammation. Even though omalizumab is generally thought to have a great safety record, there are some major concerns about the medication’s potential side effects (4). Patients using omalizumab occasionally reported experiencing headaches, nausea, or fatigue. Despite the fact that omalizumab is an antibody that is not anaphylactogenic, anaphylactic episodes have been documented. Patients with asthma who were given omalizumab compared to those who received a placebo experienced a small increase in malignancies. Omalizumab medication has occasionally been linked to a few episodes of Churg-Strauss syndrome. Some studies have linked omalizumab treatment to a moderate rise in the prevalence of helminthic infections (5).
Omalizumab is a IgG-class antibody (6).
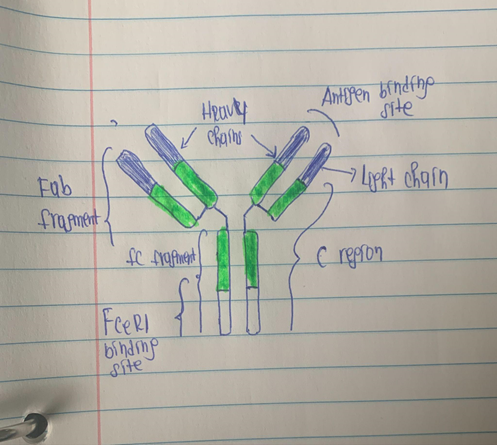
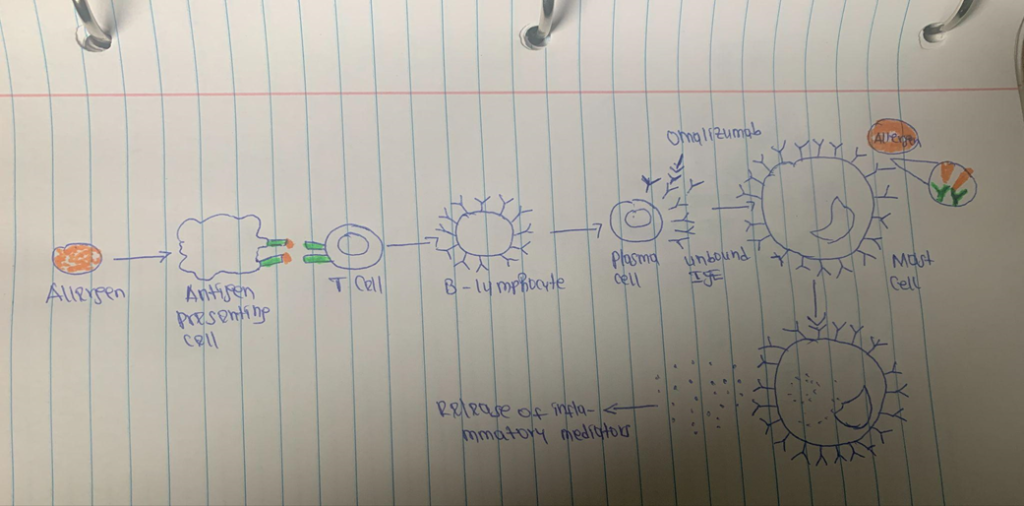
To cause an allergic reaction, antigen-specific IgE attaches to high-affinity receptors on mast cells and basophils. This causes a variety of inflammatory mediators, such as histamine to be released. Additional cells that cause inflammation, such as dendritic cells, eosinophils, and monocytes, exhibit high-affinity receptors. Omalizumab antibody that binds to the FC area of the IgE immunoglobulin. Omalizumab lowers the amount of free IgE and blocks IgE from attaching to high-affinity IgE receptors on mast cells and basophils. Treatment with omalizumab also decreases the expression of FCɛRI receptors on basophils and mast cells. By lowering the production of high-affinity IgE receptors on cells that cause inflammation and the amount of eosinophils in the airways, omalizumab reduces allergic airway inflammation (7). Omalizumab also functions as a neutralizing immunoglobulin by attaching IgE at the same location as the high-affinity receptor (FCɛRI). As a result, IgE effector functions are suppressed, which in turn prevents the reaction of mast cells and basophils. Omalizumab decreases the frequency of asthma symptoms by stopping the cellular response from activating (8).
References
- Stokes, J. (2017, September 21). Anti-ige treatment for disorders other than asthma. Frontiers in medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5613080/#S2title
- U.S. Department of Health and Human Services. (n.d.). What is asthma?. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/asthma
- Genentech: Press releases: Monday, Apr 12, 2021. Genentech: Press Releases | Monday, Apr 12, 2021. (n.d.). https://www.gene.com/media/press-releases/14904/2021-04-12/fda-approves-xolair-omalizumab-prefilled
- Kumar, C., & Zito, P. M. (2023, August 27). Omalizumab – StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK545183/
- Pelaia, C., Calabrese, C., & Pelaia, G. (2018, November 7). Omalizumab, the first available antibody for biological treatment of … https://journals.sagepub.com/doi/10.1177/1753466618810192
- Gon, Y., Maruoka, S., & Mizumura, K. (2022, March 10). Omalizumab and IGE in the control of severe allergic asthma. Frontiers in pharmacology. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8960644/#:~:text=This%20is%20attributed%20to%20the,Wu%20and%20Zarrin%2C%202014).
- Thomson, N. C., & Chaudhuri, R. (2012, June 12). Omalizumab: Clinical use for the management of asthma. Clinical medicine insights. Circulatory, respiratory and pulmonary medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3382304/
- D’Amato, G., Salzillo, A., Piccolo, A., D’Amato, M., & Liccardi, G. (2007, August). A review of anti-ige monoclonal antibody (omalizumab) as add on therapy for severe allergic (IGE-mediated) asthma. Therapeutics and clinical risk management. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2374942/#b5

Leave a Reply