Rituximab
Mark Ciardiello
Bachelor of Science
BIOL302 Introduction to Immunology
2/16/2024
Rituximab is a monoclonal antibody that treats autoimmune conditions involving CD-20
positive B-cells, with a major condition being CD20 positive B-cell Non-Hodgkin’s Lymphoma
(4). Non-Hodgkin’s Lymphoma is a cancer that is found in the lymphatic system. The cancer is
known to be both slow and aggressive, with the most aggressive type having a very easy way to
spread. The lymphatic system is connected across the entire body, with lymph nodes scattered
throughout, giving places for lymphomas to reside. Non-Hodgkin’s Lymphoma becomes
extremely deadly when it starts to spread to other organs around the many lymph nodes it can
reside in while it grows (1). Rituximab aims to target the very B-cells that are causing Non-
Hodgkin’s Lymphoma. This monoclonal antibody was developed by Genentech and approved by
the FDA in 1997 (2)(3). The FDA has approved two official dosing regimens. The first regimen
details a single IV infusion of 375mg/m2 weekly for 4-8 consecutive weeks on its own or
alongside other chemotherapy drugs. The second regimen involves two IV infusions of 1,000mg
given two weeks apart, while methotrexate is being administered alongside (2). The side effects
of rituximab vary, with some mild and some being severe. The most common side effect is an
allergic reaction or other type of reaction to the initial infusion of rituximab. Reactions typically
occur within the first 24 hours of the first infusion. The mild symptoms include fever, chills,
rash, while the severe symptoms may include shock, anaphylaxis, and even death. Due to the
nature of rituximab having immunosuppressant effects, increased infection risk exists with the
application of rituximab (4).
Rituximab works by first identifying CD-20 positive B-cells. It binds to CD-20 through
its heavy and light chain variable regions that specifically target CD-20. The IgG monoclonal
antibody also has both a human IgG 1 and kappa-chain constant region. These regions allow the
rituximab to bind to effector cells like macrophages and neutrophils, which mediate antibody-dependent and complement dependent cytotoxicity. With both CD-20 positive B-cells and
effector cells being bound together by rituximab, cell death of these B-cells will be the result (3).
The benefit of targeting cells with CD20 expression is that it avoids the targeting of mature
plasma cells, which are responsible for producing immunoglobulins, a very important class of
glycoproteins (2).
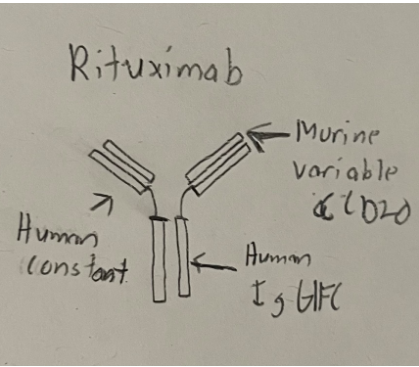
(5).
The end goal of administration of rituximab is to improve the health of the patient that it
is being administered to. With the health of the patient being deteriorated by the activity of CD-
20 positive B-cells, the regulation of these cells is the goal. Rituximab helps to bridge the
harmful B-cells with effector cells to regulate B-cell apoptosis, effectively reducing the
symptoms found in the autoimmune diseases caused by B-cells.
References
1. Connors, J. (2013). Non-Hodgkin lymphoma: the clinician’s perspective—a view from
the receiving end. Mod Pathol 26 (Suppl 1), S111–S118.
https://doi.org/10.1038/modpathol.2012.184
2. Emer JJ, Claire W. (2009). Rituximab: a review of dermatological applications. J Clin
Aesthet Dermatol, 2(5):29-37. PMID: 20729962.
3. Hanif N, Anwer F. (2022). Rituximab. StatPearls Publishing,
https://www.ncbi.nlm.nih.gov/books/NBK564374/.
4. Kasi PM, Tawbi HA, Oddis CV, Kulkarni HS. (2012). Clinical review: Serious adverse
events associated with the use of rituximab – a critical care perspective. Crit Care,
16(4):231. https://doi.org/10.1186/cc11304.
5. Smith, M. (2003). Rituximab (monoclonal anti-CD20 antibody): mechanisms of action
and resistance. Oncogene 22, 7359–7368. https://doi.org/10.1038/sj.onc.1206939

Leave a Reply