Personal Scientific Literacy Paper
Personal Scientific Literary Paper
Martin Ensinger
December 9, 2018
Prof: Dr. Christina Steel
BIOL 293 Section: Online
Coming from an environmental science background with an interest in microbiology and bioremediation, I chose a topic on the study of redox potential stress and it’s role in cell growth. In controlling the metabolic state of aerobic bacterial cultures. This is an area of interest in understanding the pathway and mechanism involved in redox sensing bacteria and the regulation of bacterial metabolism. With attention on how redox reagents may affect the function of ATPase in bacteria that can sense and respond to redox stress. The use of bacteria for bioremediation and bioremediation has become more practical and feasible. Ghazaryhan team chose to study the redox stress of Geobacillus, a thermophiles bacteria that is found in high temperatures 40-75 C and in acidic environment of 6.0 -8.5 pH (Nazaine et al., 2001).
Genus Geobacillus bacteria is a gram-positive bacteria capable of; aerobic respiration, facultative anaerobic respiration if oxygen is present, and fermentation respiration if oxygen is absent. Geobacillus toebii is classified as a thermophile as optimal growth occurs at high temperatures of 45-70 C. It does have flagella for motility can appear non-motile. Growth can be driven by aerobic or anaerobic respiration, using a large variety of redox pairs. (Sung et al, 2002) Different species of the Genus Geobacillus and other thermophiles have been studied for possibly use in hydrocarbons and the oil industry due their unique enzymes and metabolic pathway (Fulazzaky, Astuit, and Fulazzaky, 2015)
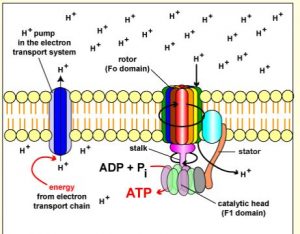
In “Chapter 5: Aerobic Respiration and the Mitochondrion” we learned that the ATPase enzyme generators ATP by the cell plasma membrane generating an H+ gradient in prokaryotic cells. In eukaryotic cells this is done in the mitochondria. By the mitochondria creating an electron gradient between inner matrix and the intermembrane space between the outer and inner membrane. This gradient is caused by moving H+ ions out of the cell via electron carriers, the electron transport chain. Making the outside of the cell more positive due the increase in H+ and the inside of the cell more negative. Making the H+ ions wanting to enter the cell via diffusion which is done by ATPase (See figure below), creating ATP. To review, a reducing agent is the loss of electrons or gaining of H+ ions, and an oxidizing agent is the gaining of electrons or removing Hydrogen. It would make sense that changing the oxidation or redaction state of a cells environment would change its ability to produce ATP. By changing the affinity of H+ ions to enter the cell membrane via ATPase or H+ reacting with the oxidation or reducing chemicals in the extracellular environment.
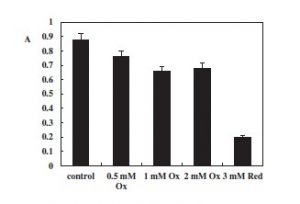
The table below shows the abundance of the growth on the y axis and concentrations of oxider Potassium Ferricyanide K3[Fe(CN)6] with glucose supplementation in all except 3mM on the x-axis of G. toebii from the study. Though there is a drop in cell abundance with Potassium Ferricyanide. It should be more pronounced and the oxidation does have an effect on cell growth as seen in the results from group 3mM Red. This is assumed to be caused by the oxiding agent affecting cell membrane transport proteins and enzymes leading to metabolism instability (Bagramtan, Galstyan, and Trchounian, 2000). For cell’s to carry out their metabolic process it is necessary for their intracellular environment to be in a reducing state, causing the H+ gradient. And for the H+ to come back in to the cell via the ATPase. Thus we see a drop in cell abundance vs the control, but not as sharp as or at steep as I would have expected. The study did mention that there was a short stimulator effect of cell abundance with the oxider though that did not last as shown by the results published.
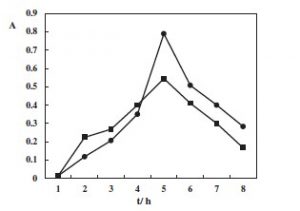
The table below showing cell abundance of the growth on the y axis and t/h on the x-axis. In the graph line ▪ represents just 3 mM of the reducing agent DL-dithionthreitol (DTT) HSCH2CH(OH)CH(OH)CH2SH and ● represents DL-dithionthreitol (DTT) with glucose supplementation of G. toebii from the study. Again there is a drop in cell abundance caused by the reducing agent alone. Even though there was an initial stimulation effect caused by reducing agent as compared to glucose. The drop in cell abundance is possibly caused by the reducing agent reducing the presence of reactive oxygen. This has been observed in Symbiobacterium toebbii another thermophile which has a symbiotic relationship with G. toebii (Kim, et al., 2011). But again I would expect more of drop in cell abundance due to the reducing agent as compared to the reducing agent and glucose.
Ghazaryan team next tested the redox and oxidizing agents compared with the ATPase inhibitor N’N’ -dicyclohexylacarbodiimide (DCCD) on G. toebii. A low H+ efflux was observed upon the addition of glucose. The addition of DCCD suppressed the H+ efflux as expected. This effect of DCCD might be explained by affecting the H+ translocation mechanism differently from F0F1-ATPase because under aerobic conditions FOF1-ATPsynthase should function in the H+ influx mode resulting in ATP synthesis (Ghazaryan, Bibulyan, Kuramistsu, and Sung, 2015). The addition of the oxidizing agent Potassium Ferricyanide almost completely inhibited H+ extrusion. Which would explain the drop in cell abundance. But did not cause complete cell death which suggest roles of different ion transport in the thermophile. The reducing agent DTT stimulated ATPase activity negligibly. ATPase activity could not be investigated under the oxidizing agent due its interfered with the enzyme assay. Though it was observed both the reducing and oxidizing agent Potassium Ferricyanide inhibited H+ extrusion. Indicating a role of ATPase in bacterial physiology and ecology, especially in redox sensing by this bacteria (Ghazaryan, Bibulyan, Kuramistsu, and Sung, 2015).
(From materials and methods: H+ and K+ fluxes where using selective electrodes. The electrode readings were outputted automatically by the LABVIEW program. Ion fluxes were expressed in mMOL/min per number of cells in a unit volume)
The optimal redox potential for different bacteria is identified based on growth regulation
upon redox stress. The addition of an oxidizer or reducing agent did reduced bacterial growth, but that the bacteria was able to compensate for the redoxing agents. This suggests an alternate redox potential (Amend and Shock, 2001) or other pathways, as suggested for E. coli or E. hirae (Poladyan, Kirakosyna, and Trchounian, 2006) and possible ATPase as part of this mechanism.
In general, the energetics of metabolic pathways of carbon sources and phosphates in thermophiles is problematic, because the energy changes observed for this class of compounds as a function of temperature currently prohibit quantitative evaluation of the energetics of many stepwise
reaction’s in metabolic pathways (Amend and Shock 2001). It should be noted that these findings for the thermophile G. toebii are quite different from those obtained for E. coli (Kirakosyan and Trchounian, 2007). Suggesting a possible different compensation due to redox stress then E. coli.
The results of this study lead to new insights into the physiology and bio-metabolism in Geobacillus and thermophilic bacteria regarding the basic characteristics of redox processes. Add to the understanding of some ecological aspects and adaptation mechanisms of microorganisms under redox stresses in extreme environments. Some changes I would make in this experiment would be to have a better control group to compare the reducing reagent with. Also present the data in the same graph format for both the reducing and oxidizing agent variables. Along with suggesting another growth stimulate besides glucose or a more natural environment for the experiment and the variable. And try to get a better measure the ATPase activity for the oxidizing agent not just the reducing. More should be done to understand how microorganisms adapt to environmental stresses and survive in harsh environments. In biology we are able to see the effect of something or how some variable changes, effecting a system or organism in a positive or negative way. But may not fully understand how it caused this changed or why it caused this change, understanding the how and why is important to our understanding of biology and biochemistry.
References
Amend, .J.P., and Shock, E.L., (2001). Energetics of overall metabolic reactions of thermophilic and hyperthermophilic archaea and bacteria, FEMS Microbiology Reviews, 25 (2), pp 175–243
Bagramyan, K., Galstyan, A., and Trchounian, A., (2000). Redox potential is a determinant in the Escherichia coli anaerobic fermentative growth and survival: effects of impermeable oxidant, Bioelectrochemistry, 51, pp151–156.
Fulazzaky, M., Astuti, D.I., and Fulazzaky, M.A. (2015). Laboratory simulation of microbial enhanced oil recovery using Geobacillus toebii R-32639 isolated from the Handil reservoir, Royal Society of Chemistry Journals, 5, pp 3908-3916
Ghazaryan, A., Blbulyan, S., Poladyan, A., and Trchounian, A. (2015). Redox stress in geobacilli from geothermal springs: Phenomenon and membrane-associated response mechanisms. Bioelectrochemistry, 105, pp 1-6
Kim, K., Kim, J.J., Masui, R., Kuramitsu, S., and Sung, M.M., (2011). A commensal symbiotic interrelationship for the growth of Symbiobacterium toebii with its partner bacterium,
Geobacillus toebii, BMC Research Notes, 4, pp 437
Kirakosyan, G., and Trchounian, A. (2007). Redox sensing by Escherichia coli: effects of copper ions as oxidizers on proton-coupled membrane transport, Bioelectrochemistry, 70, pp 58–63.
Nazina, T.N., Tourova, T.P., Poltaraus, A.B., Novikova, E.V., Grigoryan, A.A., Ivanova, A.E., Lysenko, A.M., Petrunyaka, V.V., Osipov, G.A., Belyaev, S.S., and Ivanov, M.V., (2001). Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans,G. kaustophilus,G. thermoglucosidasius andG. thermodenitrificans, Int. J. Syst. Evol. Microbiol, 51, pp 433–446.
Poladyan,.A., Kirakosyan, G., and Trchounian, A., (2006). Enterococcus hirae growth and proton potassiumexchange: protonophore effect and role of oxidation–reduction potential,
Biophysics, 51, pp 499–503.
Sung, H.M.,, Kim, H., Bae, J.W.,, Rhee, S.K., Jeon, C.O., Kim, K., Kim J.J., Hong, S.P., Lee, S.G., Yoon, J.H., Park, Y.H., and Baek, D.H., (2002). Geobacillus toebii sp. nov., a novel thermophilic bacterium isolated from hay compost, International Journal of Systematic and Evolutionary Microbiology, 52, pp 2251-2255.