
The Structure of Lipid Biomolecules: A Deeper Dive
Lipids are some of the most important biomolecules in living organisms, playing essential roles in energy storage, cellular membranes, and signaling. But what makes lipids so unique is their structure. Unlike proteins or nucleic acids, lipids have a variety of structural forms that allow them to perform their diverse functions in the body. In this post, we’ll take a closer look at the structure of lipid biomolecules, breaking down their components and how they’re built for specific biological purposes.
What Exactly Are Lipids?
Lipids are a broad class of hydrophobic (water-repelling) biomolecules that include fats, oils, phospholipids, steroids, and more. While they vary in structure, all lipids share one common characteristic: their inability to dissolve in water. This hydrophobic property is what gives them their versatility, particularly when it comes to forming the membranes that encase cells.
Let’s explore the structures of the major types of lipids.
- Fatty Acids: The Building Blocks of Lipids
Fatty acids are the simplest form of lipids and serve as the foundation for more complex lipids. Structurally, fatty acids are made up of two parts:
Hydrocarbon Chain: This is a long chain of carbon atoms bonded to hydrogen atoms. The length of the chain can vary, typically ranging from 12 to 24 carbon atoms.
Carboxyl Group (-COOH): At one end of the hydrocarbon chain, there is a carboxyl group, which contains both a carbonyl group (C=O) and a hydroxyl group (OH). This end is polar and hydrophilic (water-attracting), while the hydrocarbon chain is nonpolar and hydrophobic.
Fatty acids are classified into two main types based on the presence of double bonds between the carbon atoms:
Saturated Fatty Acids: These fatty acids have no double bonds between carbon atoms. The lack of double bonds allows the molecules to pack tightly together, making them solid at room temperature (e.g., palmitic acid, commonly found in animal fats).
Unsaturated Fatty Acids: These fatty acids contain one or more double bonds, which introduce kinks into the chain and prevent tight packing. As a result, they are liquid at room temperature (e.g., oleic acid, found in olive oil).
- Triglycerides: The Body’s Energy Storage
Triglycerides are the most common form of lipid in the body and are primarily used for energy storage. They consist of three fatty acid molecules linked to a glycerol molecule. Glycerol is a three-carbon molecule with hydroxyl groups (-OH) attached to each carbon.
Structure: A triglyceride consists of a glycerol backbone and three fatty acid chains attached via ester bonds. Each ester bond is formed by a reaction between the hydroxyl group of glycerol and the carboxyl group of a fatty acid.
Function: Triglycerides are stored in fat cells (adipocytes) and serve as a dense source of energy, providing more energy per gram than carbohydrates or proteins.
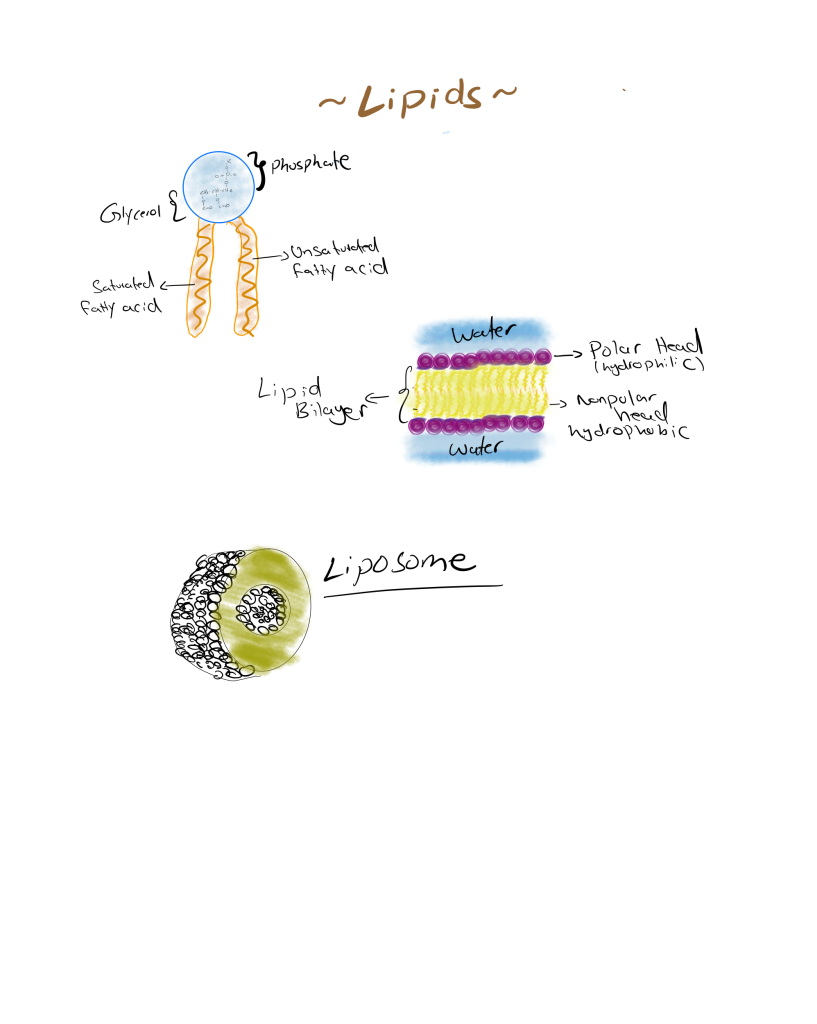
- Phospholipids: The Architects of Cell Membranes
Phospholipids are essential for the structure of cell membranes. They share a similar structure to triglycerides, but with one key difference: instead of three fatty acids, they have only two, and the third position is occupied by a phosphate group.
Structure: A phospholipid molecule has a glycerol backbone, two fatty acid tails, and a phosphate group attached to the third position. The phosphate group is often further linked to other molecules like choline, serine, or ethanolamine.
Amphipathic Nature: Phospholipids are amphipathic, meaning they have both a hydrophobic (water-repelling) tail made of fatty acids and a hydrophilic (water-attracting) head made of the phosphate group. This unique structure allows phospholipids to arrange themselves into bilayers in aqueous environments, forming the fundamental structure of biological membranes.
In a membrane, the hydrophobic tails face inward, shielded from water, while the hydrophilic heads face outward, interacting with the aqueous environment. This arrangement creates a semi-permeable barrier that controls the movement of substances in and out of the cell.
- Steroids: The Four-Ring Structure
Steroids are lipids with a completely different structure from triglycerides and phospholipids. Steroids have a four-ring carbon structure that is fused together, making them rigid and planar molecules.
Structure: The core structure of steroids consists of four fused carbon rings. These rings are labeled A, B, C, and D, and various side chains or functional groups are attached to these rings, depending on the specific steroid.
Cholesterol: The most well-known steroid is cholesterol, which is a crucial component of cell membranes. Cholesterol helps maintain the fluidity of the membrane, preventing it from becoming too rigid or too fluid.
Steroid Hormones: Cholesterol is also the precursor to steroid hormones like testosterone, estrogen, and cortisol, which regulate processes like metabolism, growth, and stress response.
- Glycolipids: Lipids with Carbohydrates Attached
Glycolipids are lipids that have a carbohydrate group attached to their structure. They are found in the cell membranes of neurons and play a key role in cell-cell recognition and communication.
Structure: A glycolipid consists of a glycerol backbone, two fatty acid chains, and a carbohydrate group attached to the third position. The carbohydrate group is hydrophilic, while the fatty acid tails remain hydrophobic.
Function: Glycolipids are involved in signaling and recognition between cells, especially in the nervous system. They help in processes like immune response and tissue development.
Conclusion: The Diversity of Lipid Structures
The structure of lipid biomolecules is as varied as their functions. From the simple, energy-storing fatty acids to the complex, functional phospholipids and steroid hormones, lipids are essential for life as we know it. Their ability to form membranes, store energy, and facilitate communication between cells makes them indispensable to cellular function and the overall health of an organism.
Understanding the structural diversity of lipids helps us appreciate their vital roles in maintaining the integrity and functionality of cells and tissues. Whether you’re studying the fluidity of cell membranes or the signaling pathways of hormones, lipids are at the core of many biological processes, and their unique structures are the key to their versatility.

Leave a Reply