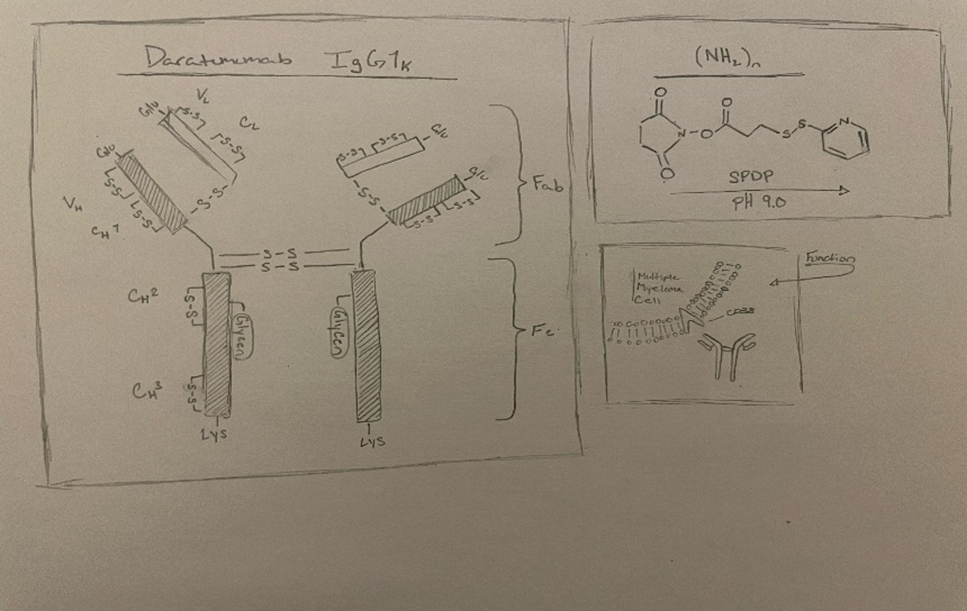
Daratumumab
Jo Cohen
Biomedical
Immunology 302
10/6/2023
Daratumumab (DARA) is a monoclonal antibody that is utilized for the therapy of multiple myeloma tumors. Daratumumab was created by Jassen Biotech and Genmab and had its first breakthrough in May 2013. It was permitted through the accelerated approval program in the USA and approved by the FDA in November 2015. (1) Multiple myeloma is a cancer that takes shape on plasma cells causing dyscrasia this can build up in bone marrow and is in 1.8% of all cancers observed in the USA. (4) Multiple myeloma is known to relapse, which is why this treatment can be implemented after a patient has been administered at least three other lines of therapy beforehand, including proteasome inhibitors and even immunomodulatory agents. (1) The approved dosage for intravenous DARA is 16 mg/kg dosage which should be administered weekly for weeks one through eight, after every two weeks from week 9-24, and every four weeks from week 25 till the disease is exterminated. (1) Dara has shown its ability to reduce the number of CLL cells within mice by 42%. The reduction in the cell number was noticeable in the mice after 2 hours of administration. (2) Although some unintended effects that were witnessed when using DARA was an increase in susceptibility to infection; this can be attributed to the destruction of NK cells which assist the innate immune system.
Targeting the Cyclin ADP ribose hydrolase (CD38) using antibodies CD38 is found in immune cells; it functions as a transmembrane glycoprotein that, when overexpressed, can cause a variety of tumor types, including myeloma (4). CD38 has also been seen in multiple types of non-Hodgkin lymphoma (NHL) and has been proven to be a great target for antibody-based therapy. (3) This further shows that, when targeted by DARA, as seen with NHL cells, when DARA binds to CD38, it effectively kills ADCC and ADCP. When DARA targets CD38, function will be inhibited, leading to a weakened immune response and metabolic disturbances. (5) Preclinical studies that have been taken of DARA have exhibited that it is active against chronic lymphocytic leukemia cells (CLL), causing a strong result of adhesion and migration on CLL cells. (1) This will allow DARA to kill tumor cells via complement-dependent cytotoxicity (CDC). (2) The use of DARA reduces adenosine manufacturing in the bone marrow; this small adjustment recreates the microenvironment, which further advances the improvement of T cell activity in the diseased area. DARA has also been observed to increase the potency of other anti-cancer drugs in their immune-stimulating degree. (5) Although many combinations are still being investigated to find what DARA can best be combined with to better stimulate the immunomodulatory effects of the CD38 antibodies.
References
1. McKeage, K. Daratumumab: First Global Approval. Drugs 76, 275–281 (2016). https://doi.org/10.1007/s40265-015-0536-1
2. Matas-Céspedes, A., Vidal-Crespo, A., Rodriguez, V., Delgado, J., Villamor, N., Roue, G., … & Pérez-Galán, P. (2014). Daratumumab, a novel anti-CD38 monoclonal antibody shows anti-tumor activity in CLL and hampers leukemia-microenvironment interactions. Blood, 124(21), 4680. https://doi.org/10.1182/blood.V124.21.4680.4680
3. Vidal-Crespo A, Matas-Céspedes A, Rodriguez V, et al (2020) Daratumumab displays in vitro and in vivo anti-tumor activity in models of B-cell non-Hodgkin lymphoma and improves responses to standard chemo-immunotherapy regimens. Haematologica. 2020 Apr;105(4):1032-1041. doi: 10.3324/haematol.2018.211904
4. Nooka, A. K., Kaufman, J. L., Hofmeister, C. C. et al (2019). Daratumumab in multiple myeloma. Cancer, 125(14), 2364-2382. https://doi.org/10.1002/cncr.32065
5. Van de Donk, N. W. (2018). Immunomodulatory effects of CD38-targeting antibodies. Immunology letters, 199, 16-22. https://doi.org/10.1016/j.imlet.2018.04.005