We use intense, nanosecond-duration electric pulses (nsEP) to stimulate, modify, damage, or interrogate living cells and tissues. We focus on cellular and molecular mechanisms of nsEP effects and their applications in research and medicine.
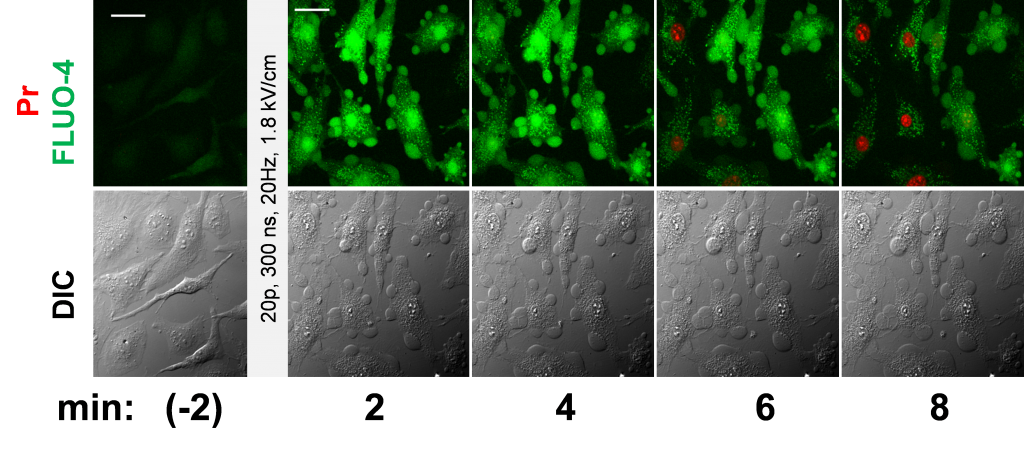
The major primary effect of nsEP is a long-lasting permeabilization (electroporation) of the cell membrane. It can be revealed by imaging the uptake of ions and dyes which do not pass through the intact membrane, as well as by measuring the transmembrane electrical current. nsEP can evoke action potentials, electroporate organelles, modify membrane proteins, activate Ca2+ and phosphoinositol signaling, and trigger a myriad of reversible and irreversible downstream effects.
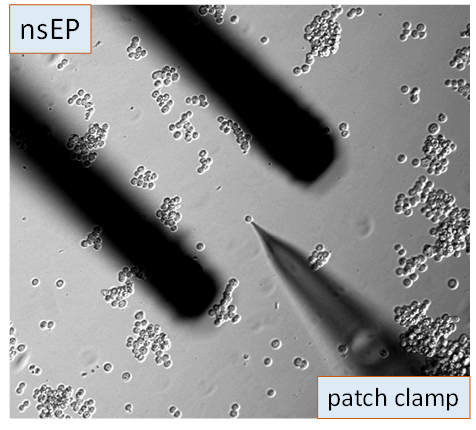
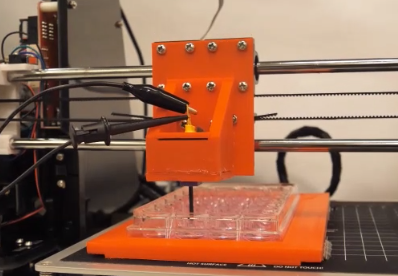
To study these effects, we have assembled three specialized, inverted microscope-based workstations equipped for a fully controlled nsEP stimulation during live cell imaging (confocal, TIRF, and ratiometric), patch-clamp recording, and synchronized with drug or media exchanges. One more workstation was custom designed for high-speed, high-resolution automated large area scanning by stitching of multiple images. We also utilize a variety of specialized devices and platforms for exposure of samples and peripheral nerve preparations to electric pulses, dosimetry, and pulse monitoring. We have recently built a 3D printer-based high-precision robotic system for electrode positioning and high-throughput treatment of adherent cells in multiwall plates.