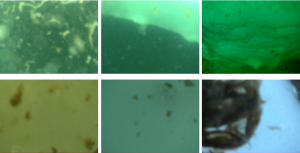
Some pictures from sidekick#3, deployed in March of 2018. You can see huge clumps of ice algae, these grow attached to the underside of the ice and then fall off as the ice melts and sink to the seabed.
Author: vhill
Daily WARM report
Here are the daily WARM figures. Have a great day [slideshow]
—
This email has been checked for viruses by Avast antivirus software. https://www.avast.com/antivirus
Watch the ice melt at WARM site #6
This is a video of the daily surface pictures from WARM deployment site six. The buoy was deployed in March of 2017 and the ice melted out in August.
Exploring the shallow water carbon pump in the Gulf of Mexico
Scientists from ODU and WHOI are at the Florida State University Coastal Marine Laboratory this summer to study the role of seagrasses in sequestering carbon in the shallow waters of the Gulf of Mexico. The team led by Drs. Zimmerman and Burdige of ODU, and Dr. Matt Long from Woods Hole Oceanographic Institution, are utilizing cutting-edge methods for measuring photosynthetic oxygen and carbon exchange (Eulerian and eddy covariance techniques) combined with sediment geochemistry measurements to build and test numerical models that can be scaled up to quantify the dynamics of carbon flux in seagrass meadows.
Photos. Upper Left: Sediment traps collect samples for analysis of carbon deposition rates in seagrass meadows. Upper Right: Our 28’ research vessel on station in St. George Sound, Fl. Lower Left: A dense meadow of seagrass Syringodium filiforme at one of our stations. Lower right: Graduate student Brian Collister preparing to collect seagrass and sediment samples using SCUBA.
Time series of daily underice photographs
Follow this link to see an video of all of the under ice photographs from our Arctic buoys for this season. At first the water looks blue, due to there being very little material absorbing light. In July the water turns the color of peak soup, this is from the presence of a phytoplankton bloom. This was very interesting as the presence of blooms under the ice has only recently been documented. After the bloom the water is again blue in color as the absorbing materials sink out to deeper waters.
Buoy 1 the journey so far
Here is a short video detailing the adventures of buoy #1 since 9th March. This is the system that we deployed North of Barrow, Alaska. I have named this buoy “Commander Vimes” if you don’t get the reference i highly recommend reading the works of Terry Pratchett.
On the ice at Barneo (North Pole Camp)
Barneo, the North Pole ice camp. Our latitude is 89N and 20E, outside temperature -27oC, you could be fooled into thinking that you are on land, but in fact under the 2 m of ice there is 2,000 m of water. Barneo is a public camp, run by Russians and populated by polar explorers and thrill seekers, plus scientists. To get here you fly through the Norwegian Spitzbergen islands, and it is here that I met up with the group from the University of Washington that operates the North Pole Environmental Observatory. A couple of days to sort through all the equipment that was shipped here over the last year, and we are ready to head to camp. It is a 2.5 hour plane ride to Barneo and we arrive at lunchtime. As I only have 3 days here I was eager to find a location for the buoy and get it deployed. We spent our first day searching the surrounding flows for a good spot. The ideal location is a flat ice pan surrounded by ridges, the fracturing of the floe will most likely occur at these ridges, meaning that as summer progresses and our floe drifts south, our buoys will hopefully stay intact in the middle of the floe. There is plenty of tea, coffee, hot chocolate and cookies available in the mess tent, and we retire there every few hours to warm up and refuel. With my satellite phone I am able to stay in contact with Pacific Gyre and keep them updated on my progress, it also enables me to call home and let everyone know that we are ok. While we are looking for our perfect buoy location, I turn on the WARM buoy GPS, so my colleagues can at least see that our camp is drifting south several miles a day. Each day the plane arrives with more adventurers and several dog teams cycle through the camp. It is always funny, as the dogs are so excited to be on the ice, they start howling when individual dogs are harnessed up, it’s as if they are worried that they will be left out of all the fun!
Sunday comes around and we spend the morning, collecting ice core samples, and measuring light profiles, we are just waiting for a snow mobile team to drag the buoy over to our location. This finally happens at about 4pm local time. We have 24 hours of sunlight so we can work at any time of the day or night. It only takes 30 minutes to unpack all the sensors and feed them down the 10 inch ice hole. After this I call Pacific Gyre to make sure that all the sensors are uploading. Back in California they can even see the crack in the ice from the below ice camera at 20 m. With the news that the buoy is working I can breathe a sigh of relief, and just like that my Arctic field season is over for this year.
While I make my way back home, the rest of the team here have several more buoys to deploy both at camp and at various locations around the pole. I am hoping that I can come back next year, now I have seen the camp, I have some ideas for experiments to conduct while I’m here.
Going to the Arctic
It’s T-1 day until I head to the Arctic toa Russian ice camp near the north pole. I will be deploying an ice teathered buoy with a string of temperature and light sensors. I will be posting video blogs to the wesbite, just look under field blogs Arctic on the top banner.
In the meantime here are a few pictures of the buoy i will be deploying.
Clams and low pH
Becky Walawender from the Department of Biology here at ODU has been monitoring the soft shell clams that were recruited into our tank this spring. Here she describes the results so far.
Soft-shell clams (Mya arenia) have fragile, thin shells made of calcium carbonate and are susceptible to breaking under the acidic conditions associated with excessive carbon dioxide. To test this, we placed 110 soft-shell clams into sediment and monitored their survival in five different pH levels (6.0, 6.5, 7.0, 7.5, 8.0). After two months, we noticed a decrease in survivorship of the clams in pH 6.0. Of the remaining 90 clams in the pH 6 tank (20 were removed for genetic analysis), 55 were confirmed dead, mortality in the other treatments was approximately 6-11 clams. The shells of the dead clams from pH 6.O became brittle and eroded through at the middle, below the umbo.
Shoots, Rhizomes and Roots, oh my!
After a year and a half of planning, construction and wiring we have finally reached the start point of our long-term seagrass ocean acidification experiment. Last week Dick and I spent two days diving in the seagrass meadow in South Bay on the Eastern shore collecting over 1 thousand seagrass shoots to fill our experimental tanks. The rest of the BORG team spent their days cleaning the tanks and awaiting our seagrass booty.
As the plants need to survive and thrive in our tanks we had to collect not just the shoots, but the rhizomes and roots as well. In the muddy sediment of South Bay this required lots of rooting around, so much so that it looked like I had been eating mudpies when I came to the surface!
- Malee planting seagrass
- Dick planting seagrass
- Our seagrass booty
- Diving in South Bay, beautiful
- Team BORG in action
It took three days to plant all of the collection, each tray now has approximately 40 shoots and each tank has between 3 and 5 trays of South Bay grass, some tanks have grasses from Washington State for a side experiment on their tolerance to our warm summers. Now we have wait for a couple of weeks to make sure that the growth rates in all the tanks are the same before we can turn on the CO2 and start the different treatments. We have managed to recruit some folks from both Biology and OEAS to run experiments in our tanks, Becky, a graduate student is going to study the clams that were recruited into the tanks during the spring and Dr Dobbs is going to look at bacterial films.
I will post more details on those experiments once they get started. In the meantime we will all be working on our tans this summer as we spend several days each week tending to our seagrass crop.
Victoria